What Is Electrodialysis — and Why Are Ion Exchange Membranes Key?
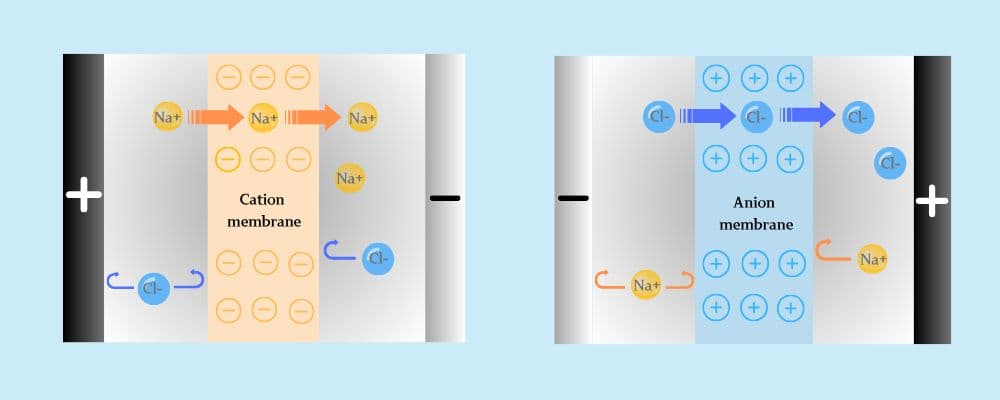
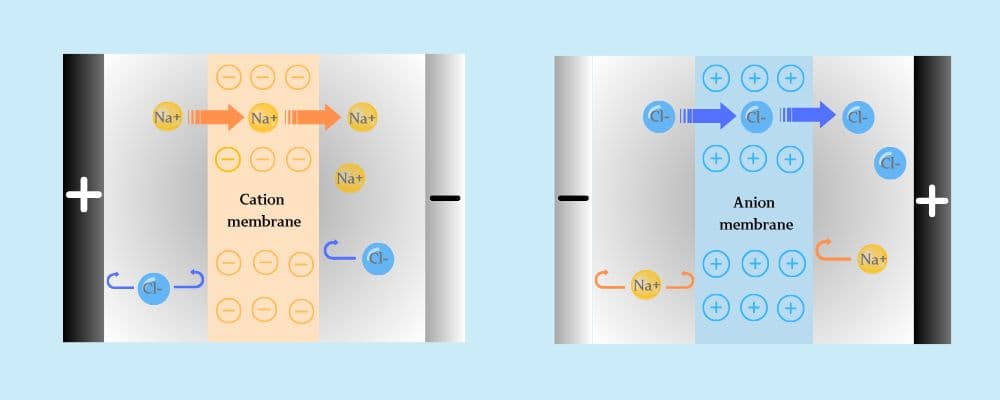
Electrodialysis (ED) is a membrane-based separation process that uses direct current and ion exchange membranes to remove ions from water selectively. It’s widely applied in desalination, concentration, purification, and resource recovery.
🔍 How it works:
• Cation exchange membranes (CEM) allow only positive ions to pass
• Anion exchange membranes (AEM) allow only negative ions to pass
• These membranes are arranged alternately between two electrodes
• Under electric current, ions migrate through the membranes, separating into concentrated and dilute streams
This highly selective ion migration enables ED systems to efficiently process saline or contaminated water with low chemical use and high precision.
Used across various industries, including food, chemicals, pharmaceuticals, power, and wastewater, electrodialysis is a reliable and scalable solution for intelligent ion separation.