Electrolysis & Electrocatalytic Oxygen
Electrolysis & Electrocatalytic Oxygen
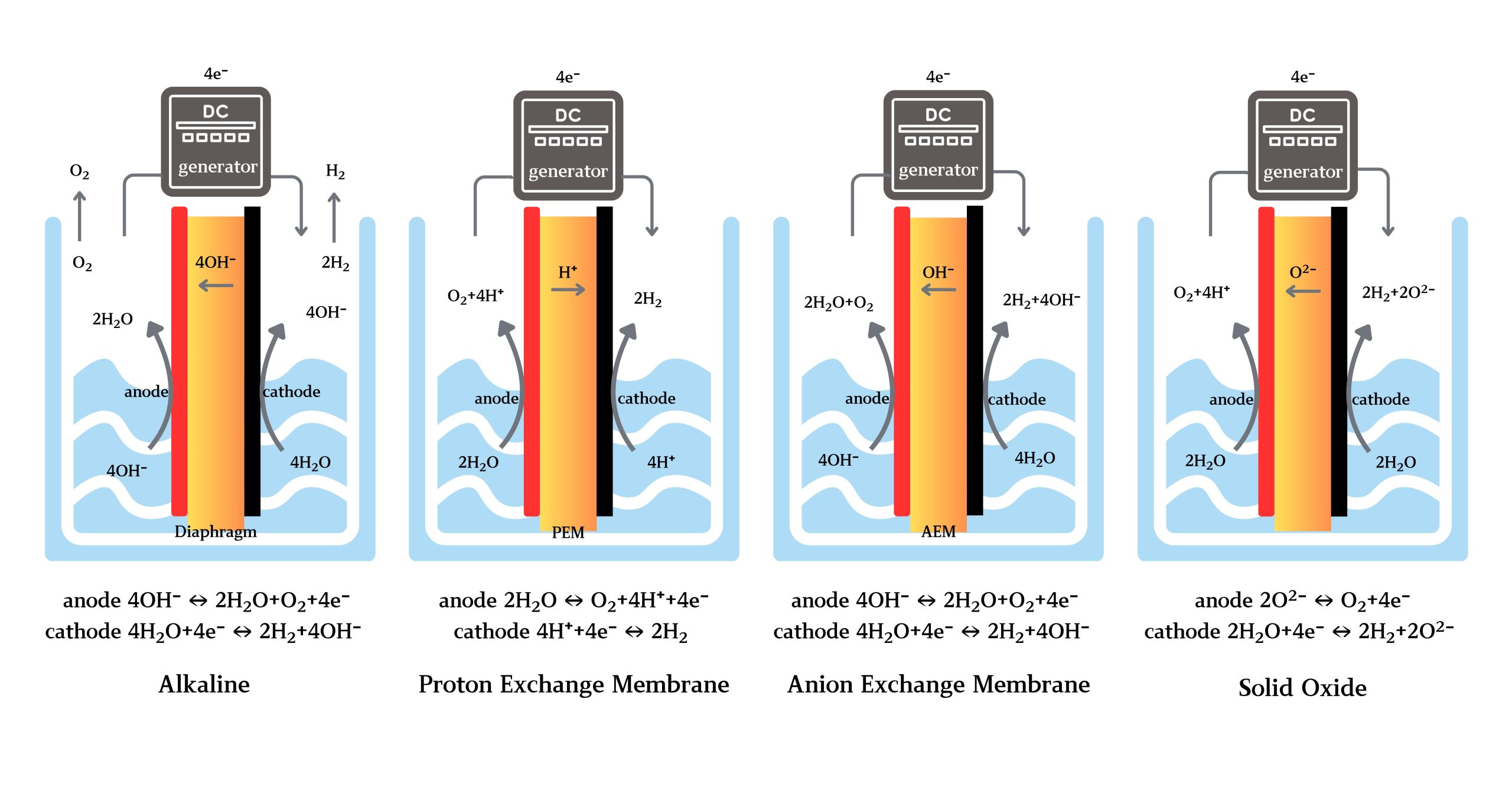
Electrolysis splits water into hydrogen and oxygen using an electrochemical process. Variants include alkaline electrolysis (ALK), proton exchange membrane electrolysis (PEM), anion exchange membrane electrolysis (AEM), and solid oxide electrolysis (SOEC). Electrolysis is critical for decarbonizing energy systems, enabling clean hydrogen production for applications in energy storage, transportation, and chemical manufacturing.
Process:
Water or reactants are fed into an electrolyzer.
Direct current (DC) facilitates ion migration and molecular dissociation.
Hydrogen, oxygen, or other products are separated and collected.
Applications:
Hydrogen production for energy and industrial use.
Chemical synthesis (e.g., hydrogen peroxide, succinic acid, oxalic acid).
CO₂ conversion to methanol or ethanol and other hydrocarbons.
Advantages:
Electrolysis produces hydrogen without emitting greenhouse gases if renewable energy is used.
High purity hydrogen and oxygen production.
Supports decarbonization across sectors like transportation, industry, and power generation.
Provides a way to store surplus electricity from intermittent renewable sources like wind and solar.
Reduces reliance on fossil fuels in chemical synthesis.