Bipolar Membrane Electrodialysis: How to Transform Lithium Sulfate into High-Purity Lithium Hydroxide?
At the final stage of lithium extraction from salt lakes or battery recycling, we often face a key challenge: how to efficiently and cleanly convert enriched lithium sulfate solution into lithium hydroxide required for battery-grade materials?
Traditional methods rely on caustic alkali (e.g., sodium hydroxide) for metathesis reactions, but this process introduces new impurities (e.g., sodium sulfate), increasing subsequent purification burdens and costs. Bipolar Membrane Electrodialysis (BPED) technology is now offering a more streamlined and environmentally friendly pathway.
A Three-Step Breakdown of the Core Principle:
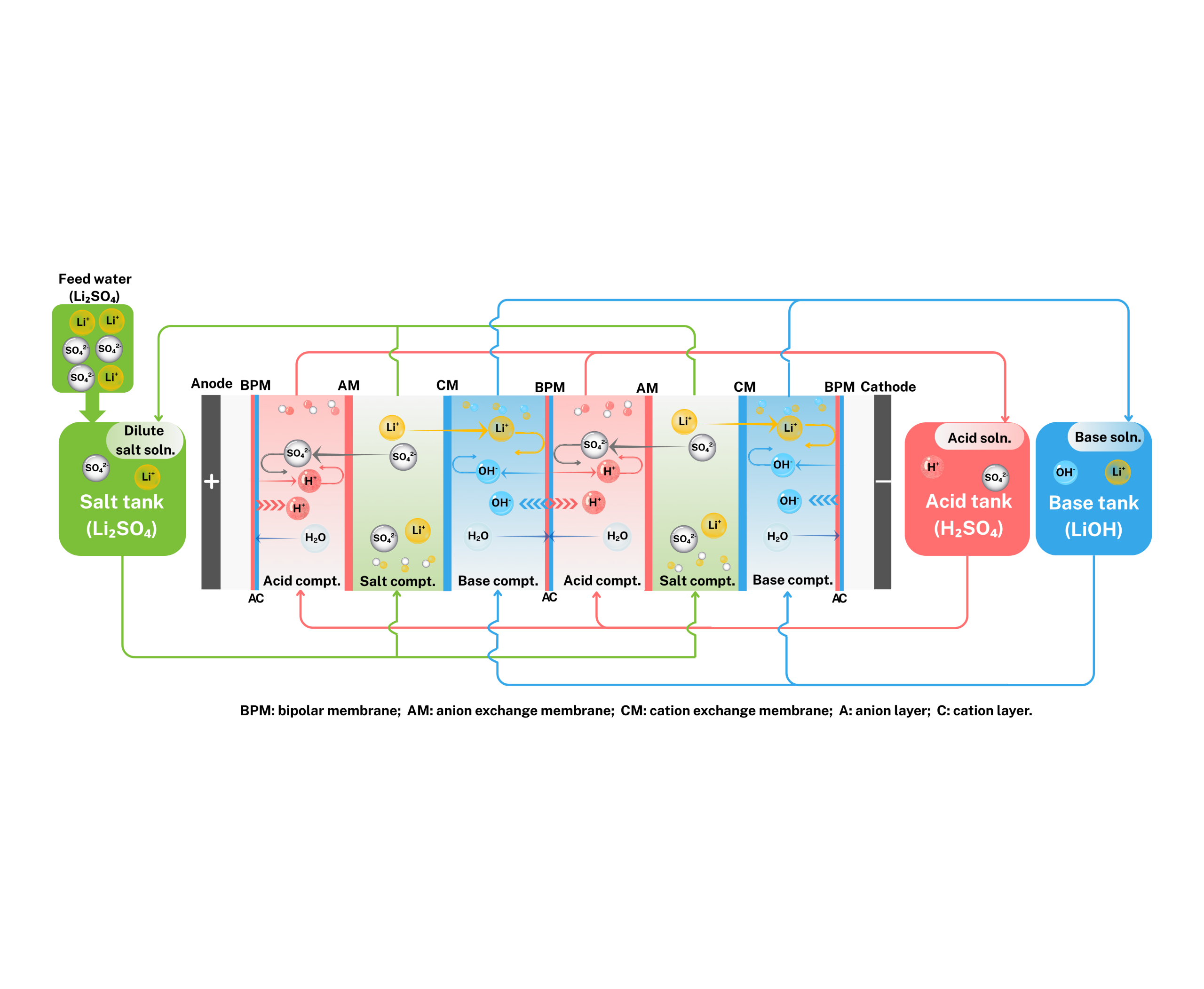
Imagine a special “magic membrane”—the bipolar membrane. It is composed of an anion exchange membrane, a cation exchange membrane, and a water dissociation catalytic layer sandwiched in between. When an electric current is applied, something remarkable happens:
Dissociation: Water molecules (H₂O) in the intermediate layer of the bipolar membrane are efficiently split into hydrogen ions (H⁺) and hydroxide ions (OH⁻) under the applied DC electric field.
Migration & Separation: Driven by the electric field, lithium ions (Li⁺) from the feed lithium sulfate (Li₂SO₄) solution migrate through the cation exchange membrane toward the cathode, while sulfate ions (SO₄²⁻) migrate through the anion exchange membrane toward the anode.
Directed Recombination: The OH⁻ generated by the bipolar membrane combines with the migrating Li⁺ in the base compartment to directly form a pure lithium hydroxide (LiOH) solution. Meanwhile, the H⁺ combines with SO₄²⁻ in the acid compartment to regenerate dilute sulfuric acid (H₂SO₄).
The Key Advantages of This Process:
No External Acids or Alkalis Needed: All required H⁺ and OH⁻ are supplied by water electrolysis, avoiding the introduction of impurity ions at the source and yielding higher product purity.
Resource Circulation: The by-product dilute sulfuric acid can be recycled back into the upstream process, enabling internal resource circulation and significantly reducing overall material costs.
Cleaner and Lower-Carbon Process: Compared to traditional methods, it reduces the generation of solid by-products, features a more compact flow, and has lower energy consumption and carbon footprint.
At Hangzhou Lanran, we have successfully implemented this technology in several salt lake resource development projects. It is not only a critical step in converting lithium sulfate to lithium hydroxide but also a core component of our integrated solution for green and efficient lithium extraction.